OUR RESEARCH
The Precision Neurotherapeutics Lab develops advanced brain monitoring techniques to better understand human brain plasticity and create more effective and personalized treatments for neurological and psychiatric disorders.
High level summary:
Our lab combines techniques from computer science, neuroscience, and engineering coupled with a clinical understanding of psychiatry and neurology to focus on three central aims:
1) To better understand the mechanisms underlying brain plasticity
2) To develop novel methods to probe the human brain
3) To personalize brain stimulation
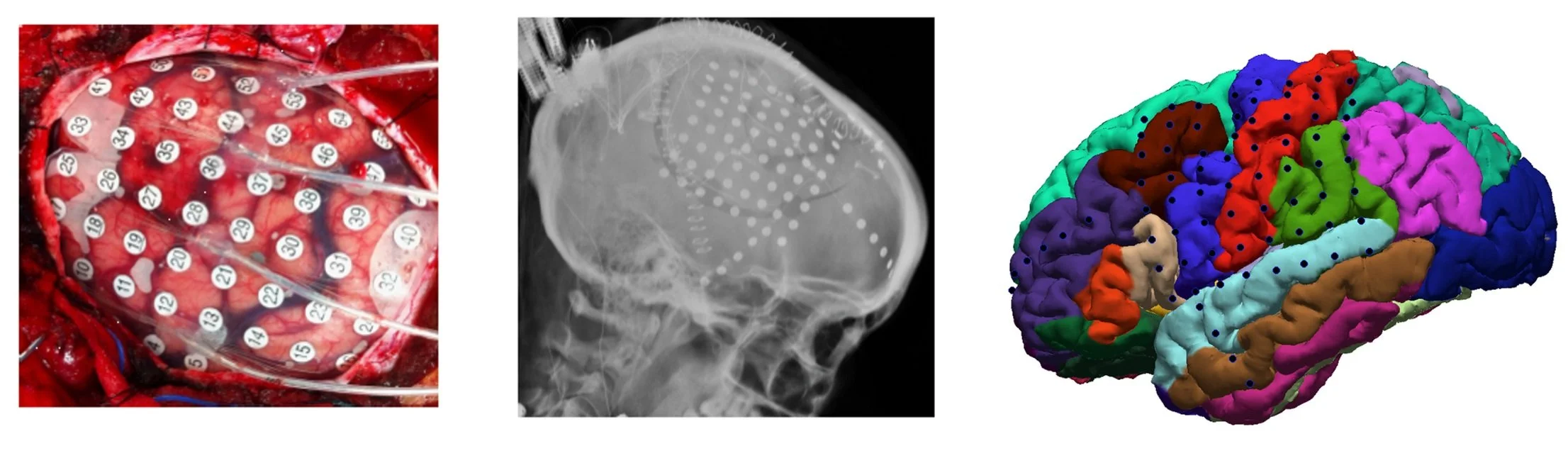
To solve these questions, we employ a multimodal approach focused on human electrophysiology. In an effort to understand mechanism as well as findings that generalize, we use a combination of invasive and non-invasive tools to stimulate and record from the human brain. Stimulation includes electrical and transcranial magnetic stimulation (TMS) while recordings span from intracranial microelectrodes measuring single or multiple neurons, to high-density scalp electrodes measuring thousands of neurons. In addition, to evaluate neuronal dynamics and brain stimulation at a more mechanistic level, we collaborate with neuroscientists using microelectrode and optogenetic tools to probe brain plasticity in animal models.
Figure 1: Methods used to probe brain activity in the lab. Left: microelectrode recordings allow single and multiunit neuronal measurements in humans and animals. Middle: combining human intracranial electrical stimulaton with intracranial EEG (iEEG) in epilepsy patients provides high spatial and temporal resolution but limited generalizability. Right: pairing TMS and scalp high-density EEG (hdEEG) in healthy controls and patients with psychiatric disorders provides generalizability but lower signal-to-noise.
THE NEURAL MECHANISMS OF BRAIN PLASTICITY
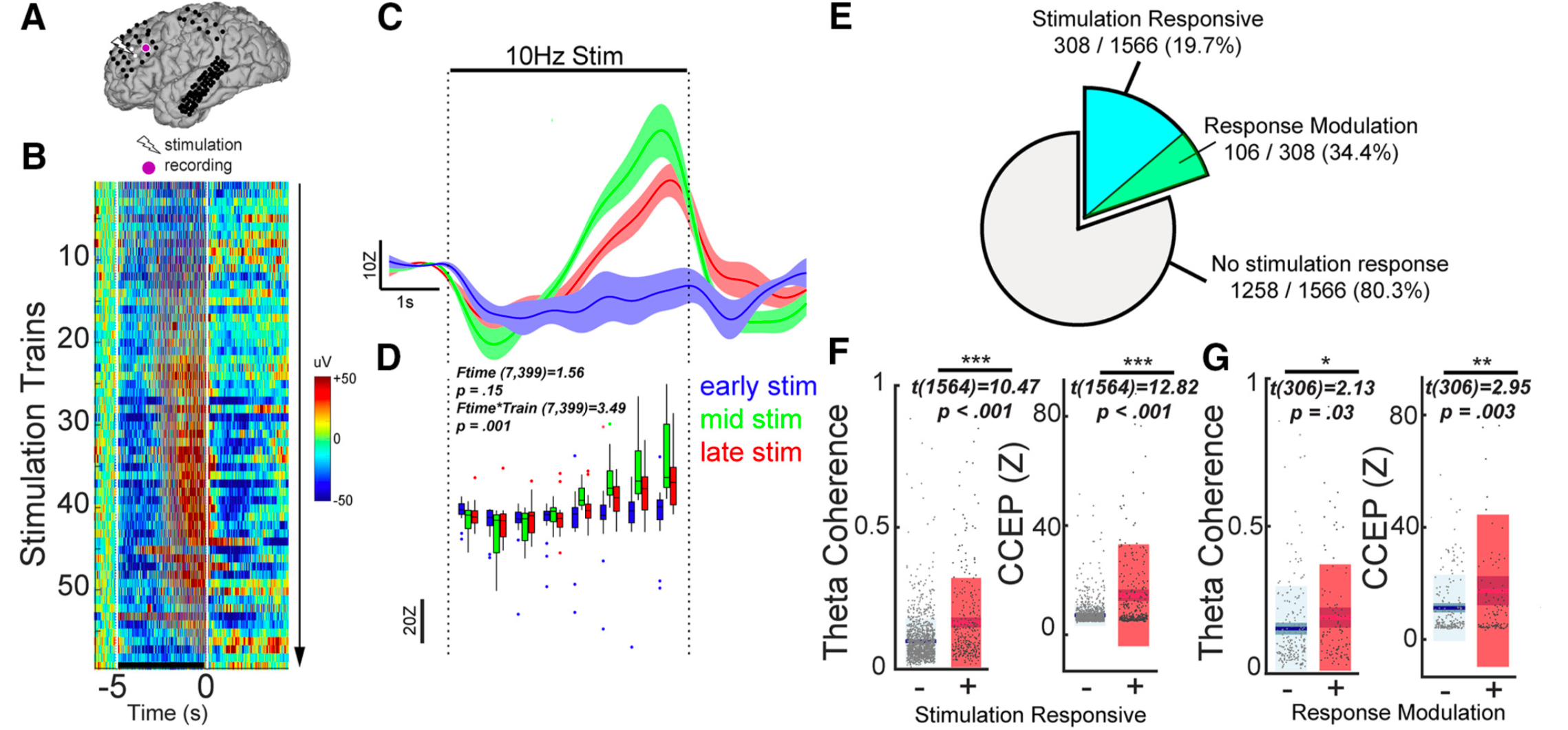
HOW DOES REPEATED BRAIN STIMULATION MODULATE NEURAL CIRCUITS?
Brain stimulation treatments including transcranial magnetic stimulation (TMS), deep brain stimulation (DBS), vagal nerve stimulation (VNS), and ultrasound are employed to alleviate neurological and psychiatric symptoms by modulating specific brain networks. However, we still have little understanding of how, where, when, and why the brain changes when it is stimulated. Developing the fundamental principles underlying brain plasticity is essential for any future optimization of these approaches. Specifically, understanding the relationship between stimulation parameters (site, frequency, pattern, phase, amplitude), baseline brain features (brain state and connectivity patterns), and the degree of brain change (amplitude, polarity, latency, specificity, and duration) is critical to develop more efficient and effective treatments. Our lab utilizes multiple invasive and non-invasive brain stimulation and recording modalities to address this question.
Keller CJ, Huang D, Honey CJ, Du V, Fini M, Lado FA, Mehta AD. Induction and quantification of excitability changes in human cortical networks. Journal of Neuroscience: 23 (2018): 5384-98.
Huang D, Herrero J, Entz L, Fabo D, Hajnal B, Mehta A, Keller CJ. Intracortical dynamics underlying repetitive stimulation predicts changes in network connectivity. Journal of Neuroscience.
Eshel N*, Keller CJ*, Wu W, Jang J, Huemer J, Mills-Finnerty C, Wright R, Ichikawa N, Fonzo G, Sphigel S, Wong M, Yee A, McTeague L, Etkin A. Global connectivity and local excitability changes underlie antidepressant effects of repetitive transcranial magenetic stimulation. 2020. Neuropsychopharmacology.
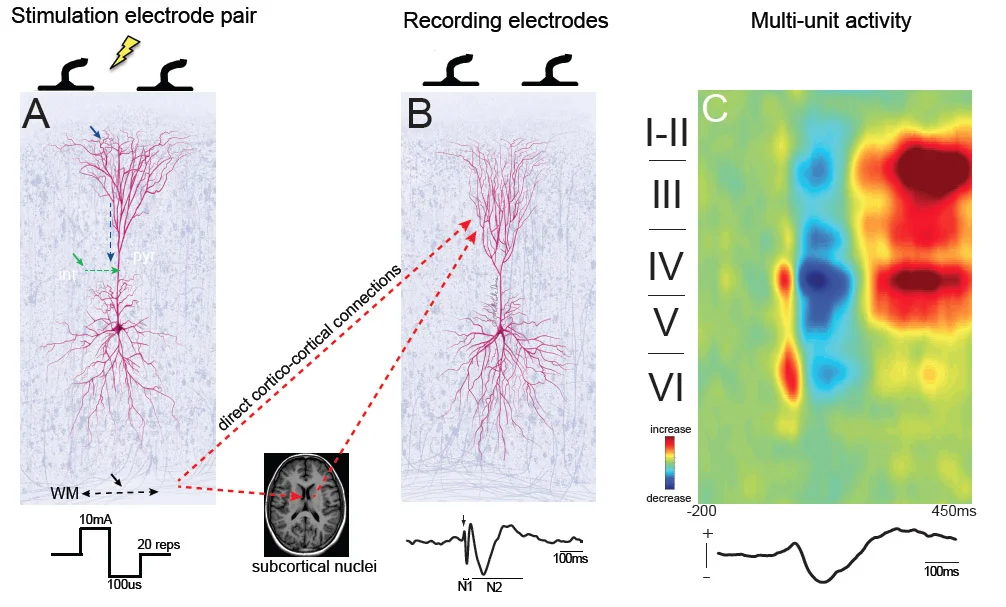
NOVEL METHODS TO PROBE THE HUMAN BRAIN
HOW CAN WE DIRECTLY MEASURE BRAIN ACTIVITY IN HUMANS?
Functional MRI and EEG have revolutionized the way in which we can measure brain activity non-invasively. However, these measures are based on statistical association and lack the causality necessary to definitively demonstrate directional relationships. Our lab develops both invasive and non-invasive methods to directly and causally probe brain circuits with high fidelity and clearer underlying mechanisms. This work will allow us to 1) individualize and optimize treatment protocols as well as 2) monitor the progression of brain stimulation interventions in real-time.
Keller CJ, Bickel S, Entz L, Ulbert I, Kelly C, Milham M, Mehta AD. Intrinsic functional architecture predicts electrically-evoked responses in the human brain. Proceedings of the National Academy of Sciences 108 (2011): 10308-13.
Keller CJ, Bickel S, Honey CJ, Groppe DM, Craddock CR, Kelley C, Lado FA, Milham M, Mehta AD. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. Journal of Neuroscience. 33 (2013): 6333-42.
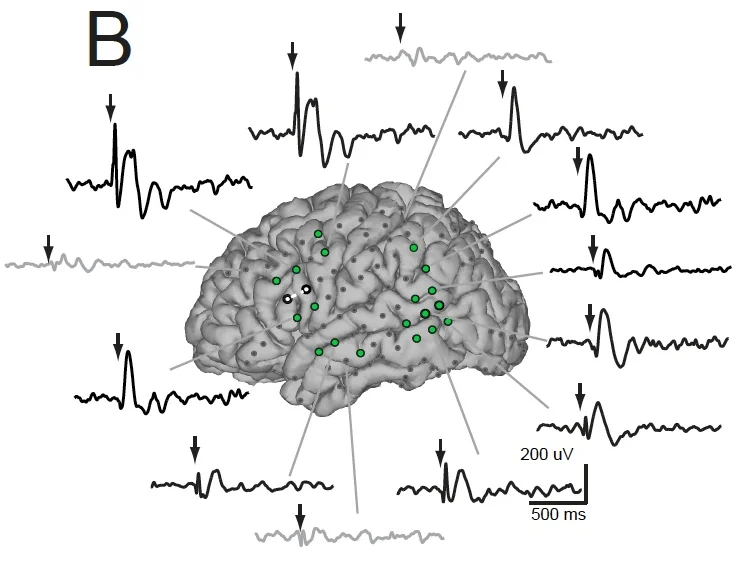
Keller CJ, Honey CJ, Entz L, Bickel S, Groppe DM, Toth E, Lado FA, Ulbert I, Mehta AD. Probing the human connectome: cortico-cortical evoked potentials reveal projectors and integrators within human brain networks. Journal of Neuroscience. 34 (2014): 9152-63.
Keller CJ, Honey CJ, Megevand P, Entz L, Ulbert I, Mehta AD. Mapping complex brain networks with cortico-cortical evoked potentials. Philosophical Transactions of the Royal Society B: Biological Sciences. 1 (2014): 369 (1653).
PERSONALIZING BRAIN STIMULATION
HOW CAN WE IMPROVE BRAIN STIMULATION TREATMENTS?
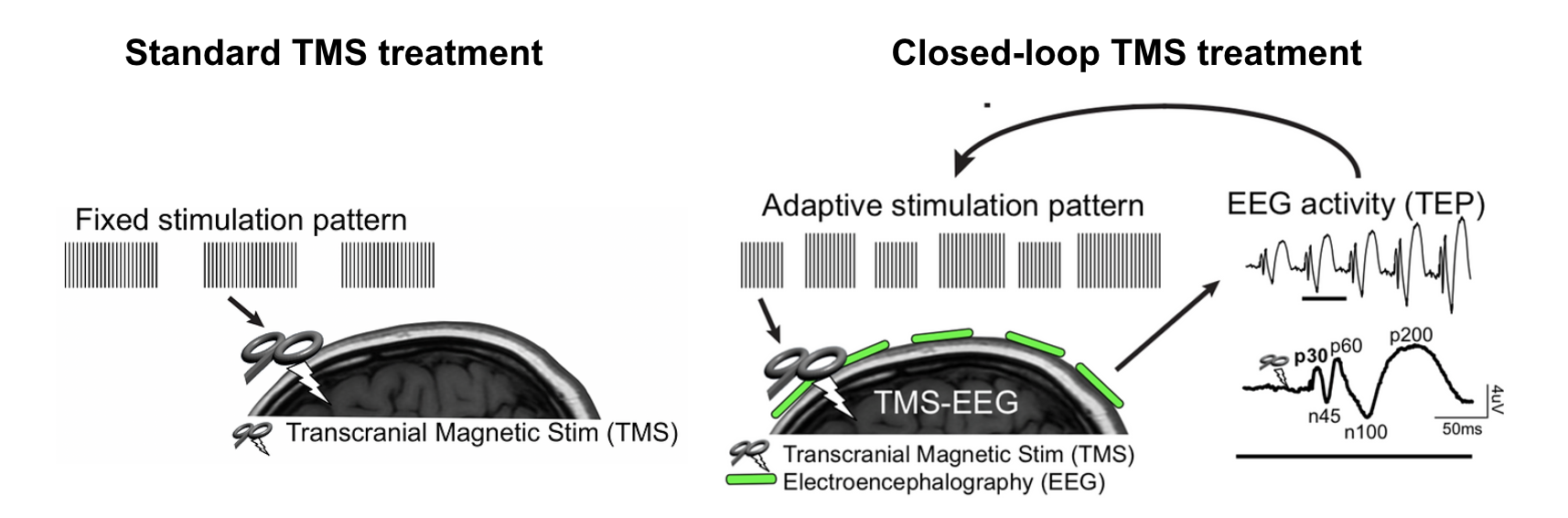
Brain stimulation treatments including TMS represent the front-line of innovative approaches to correct dysfunctional brain networks for patients suffering from mental illness. However, as currently administered, TMS is applied in a one-size-fits-all treatment for all psychiatric disorders. As a result, treatment response is <50%. We aim to develop a non-invasive, closed-loop brain stimulation platform for neuropsychiatric disorders that will more effectively treat the brain. Specifically, we will create a real-time system for monitoring brain changes, evaluate the mechanism underlying these brain changes, and develop and test an adaptive stimulation paradigm to maximally drive individual brain changes and improve clinical outcome. Successful implementation of this work includes the early stratification of treatment responders and personalized and more effective treatments for non-responders. This approach is broadly applicable to other depression biomarkers, all psychiatric populations treated with TMS, and other brain stimulation modalities.
STATE-DEPENDENT NEUROMODULATION
While TMS is an effective treatment for depression, current protocols apply stimulation in a "one-size-fits-all" manner, contributing to variable response rates of only ~50%. Our lab is pioneering a fundamentally different approach: leveraging the brain's natural states to optimize stimulation timing and enhance treatment efficacy. We have discovered that the brain's responsiveness to TMS varies dramatically depending on its ongoing activity patterns, and that timing stimulation with specific brain states can amplify neural responses by up to 75%. We are investigating three clinically feasible approaches to create optimal brain states during TMS: sensory entrainment using musical beats to synchronize neural oscillations, cognitive engagement through interference tasks that activate prefrontal circuits, and NREM sleep that harnesses natural plasticity processes. Using a combination of intracranial recordings in neurosurgical patients and high-density EEG in healthy and depressed individuals, we are mapping how these brain states influence both immediate neural excitability and lasting plasticity in depression-relevant circuits. This state-dependent approach represents a paradigm shift from treating the brain as a passive target to working with its endogenous activity patterns, offering a path to more personalized and effective neuromodulation treatments that can be implemented with minimal modifications to existing clinical protocols.
THE NEURAL BASIS OF AFFECTIVE STATES: PAIN, EMOTION, MOOD
Understanding how internal mood states relate to observable emotional expressions represents a fundamental challenge in neuroscience with critical implications for developing objective biomarkers of mental health. Our lab is pioneering the use of simultaneous intracranial EEG recordings and naturalistic behavioral monitoring to decode both facial expressions and mood states from the same high-resolution neural signals over multiple days. Using continuous video recordings from epilepsy patients, we developed FaceDx, a computer vision pipeline that automatically detects and quantifies facial expressions, speech patterns, and other affective behaviors in real-world clinical settings. We have successfully applied this approach to decode acute pain states, demonstrating that naturalistic facial expressions can reliably predict subjective pain intensity through neural activity in the anterior cingulate cortex and that pain-predictive facial features are neurally distinct from emotional expressions. We are now extending this methodology to investigate the fundamental relationship between observable facial expressions and internal mood states, examining whether these phenomena share common neural substrates or operate through distinct mechanisms. Through continuous monitoring of both spontaneous and task-based emotional states and self-reported mood over multiple days, we are mapping the neural circuits underlying momentary emotional expressions versus sustained affective states, while using direct electrical stimulation to establish causal relationships between limbic activity, facial behaviors, and internal emotional experience. These findings challenge longstanding assumptions about expression-mood relationships and suggest that effective psychiatric biomarkers will require mood-specific neural signatures rather than relying on observable behavioral proxies, paving the way for more precise and personalized approaches to mental health assessment and treatment.